Business Areas:
Bulk Drug Intermediates:
At lavybens pharma, we are dedicated in developing top of the line pharmaceutical products while adhering to highest quality norms by well qualified and renowned research team. The continuous strive for perfection has led us to push our standards of manufacturing by building state-of-the-art facilities.
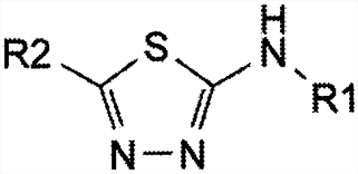
The R&D division and kilo lab facility of the bulk drug intermediates manufacturing unit devotes all its energies and is constantly engaged with the exploration and development of bulk intermediates molecules with multi-step chemical synthesis. key focus areas include
We are the long associated and manufacturing sites located in hyderabad.
We are the manufacturing bulk intermediates and supporting to several regulatory companies in INDIA
Pharmaceutical Impurity Reference Standards:
Lavybens pharma to provide analytical reference standards/impurity standards/working standards with fully characterized products manufacturing and supply of the global presence.
Reference standards are completely certified and qualified.
Maintained the regulatory guidance (US,EP,BP,JP,IP WHO and ICH and qualify the products like intermediates/impurities/reference standards and working standards.
Maintained the storage and expiry of the product as per stability guidelines.
Maintained Material Safety Data Sheet (MSDS) for each impurity / intermediates.
R&D:
Lavybens pharma has a long-term commitment to developing of advanced intermediates pharmaceutical reference standards/impurity profile for the world class research and high-quality standards.
Our research and development team consists of over 80 scientists and developers who are highly qualified and experienced in custom synthesis/process R&D/CRO/ new intermediates and analytical development.
Manufacturing of impurities, intermediates & CRO molecules
Introducing intermediates development & validation of analytical development businessin domestic market & regulatory.
Commencement of new CRO molecules development, analytical development and marketing business for regulatory & non-regulatory territories.
Process development lab:
- Process development R&D
- Process development
- Impurity Profiling
- Process engineering
- Analytical Method Development
- Scale-up
- Commercialization
Quality manufacturing:
- Validation batches
- Multi-scale manufacturing