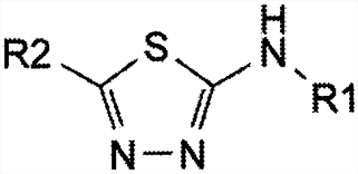
Impurity standards will be supplied along with the following documentation:
- Certificate of analysis
- Structure elucidation report.
- Infrared spectrum
- Mass spectrum
- 1H NMR spectrum
- 13C NMR spectrum
- TGA report
- HPLC chromatogram
- Additional data, if any required for customers.